Computers instead of bodies: Virtual patients replace animal and human experiments
Ronja Schrimpf
Bringing new products to the market is always associated with time and money. In the clinical field, however, these costs and timescales can take on almost unimaginable proportions. Ten years to approval and costs of around 100 million euros - and even more - can be expected for high-risk products. In addition, there are also ethical questions that arise in the performance of animal experiments.
These difficulties are history, at least if the startup Virtonomy is anything to go by. The solution is a data-driven, virtual patient, which should make animal and human experiments superfluous. In an interview with 5-HT, co-founder and CEO Dr. Simon Sonntag shows the advantages of a virtual patient and how Virtonomy aims to revolutionize clinical trials.
 Virtonomy Team
Virtonomy TeamThe classic way: try and error on humans and animals
"The classical way of product manufacturing in the clinical area is very iterative and much associated with try-and-error. This means that a specific medical device or design is developed, tested on animals and then adapted. Then the product goes back into animal testing, is adapted again and so on," explains Simon Sonntag, co-founder and CEO of Virtonomy. He originally comes from the field of applied mathematics. In the meantime, he has been working in medical technology for 10 years, including about six years with another start-up in the industry, "This iterative process is extremely expensive and lengthy.
For high-risk products, for example, the time to approval is more than ten years, with costs of over 100 million euros or even more. These are immense costs, which are mainly incurred during clinical trials.
The U.S. Food and Drug Administration (FDA) expects to save many millions of dollars for medical device manufacturers if the risk of an error in the try-and-error process can be reduced by only ten percent. In the long term, Virtonomy wants to reduce the risk by more than ten percent. But how can the classic approach be avoided at all?
The trend-setting way: Simulations on virtual patients
"Even before a product is manufactured, we can run simulations and thus carry out virtual tests on the population. This enables us to perform design iterations before the product has even been manufactured. This allows all further steps of animal and human experiments to be planned better and more precisely. It also reduces the risk of a product failing in animal or human trials," explains Simon.
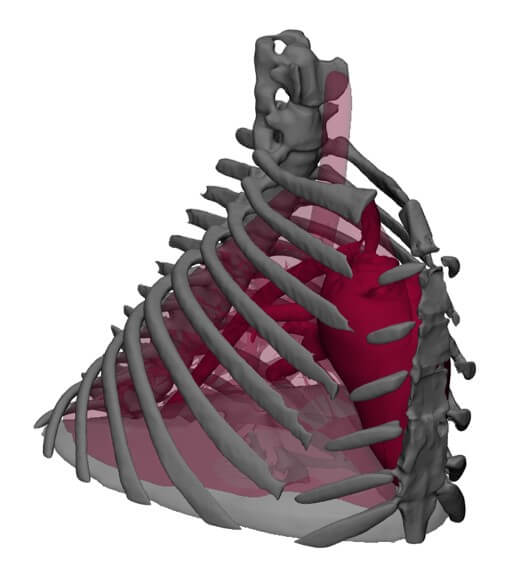
For this purpose, Virtonomy relies on a data-driven virtual patient, consisting of large datasets of clinical data that can be used to reconstruct virtual patient models.
"The virtual patient can be put together directly by the user, with all the requirements necessary for the medical device in question. The user can then carry out simulations, analyses and statistical evaluations and run through various product cycles.
This means that we can perform tests that could previously only be carried out using classical methods in animals and humans - and can thus reduce animal and human experiments". And that, Simon reveals, has a lot of advantages.
Virtonomy's mission: End human and animal experimentation
The classic route in the clinical test and approval process raises a lot of ethical questions: It requires a lot of animal testing. Large population groups are evaluated with products that have not yet been sufficiently tested, but at the same time many population groups are underrepresented in the clinical test procedure, including women and children.
Virtonomy's virtual patient enables considerable savings in time and cost, but also solves some ethical problems. Due to the amount of clinical data sets, simulations can also be performed on otherwise underrepresented population groups. And the virtual patient makes it possible to reduce the amount of necessary testing in both animals and humans.
"We have already been able to prove this with a Swedish manufacturer of an artificial heart, among others: The artificial hearts had failed in animal experiments. With our virtual animal models, we were able to virtually prepare the next series of animal experiments. In fact, the next series of animal experiments that was carried out was successful. The number of animal experiments initially set at seven could thus be reduced to three. "As a result, we have already been able to save a number of animals in this process," concludes Simon.
"Over the next five to ten years, the goal is to reduce and increasingly replace animal and human experiments," explains Simon. "Our mission is to end the use of animals and humans in clinical trials.
Virtonomy's story: young, but already successful
Virtonomy sees itself as a B2B company that addresses manufacturers of medical devices. Hospitals are not among Virtonomy's target group, but they are interesting as cooperation partners and possible data sources for the startup.
"We work closely with clinics. We rely on mutual support and exchange and can also help the clinics by analyzing certain data," says Simon. A good data basis is crucial for the data-driven virtual patient, but Virtonomy has already created a good basis.
Currently the software is still in development. However, the first version of the software should be completed by the end of the year and then published. Despite this early stage of development, Virtonomy already has a number of international customers, has already been able to acquire a number of public funds, and is in the process of completing a major investment round. And this despite the fact that the startup is only one year old.
"During my time at a startup in medical technology, I came into contact with all the regulatory requirements and was also part of an ISO committee for artificial heart valves. I saw a great opportunity in this field, a lot of growth and also a great interest of the regulatory authorities in changes," says Simon, "That's why I decided to start my own business at the beginning of 2019. After some searching, I found my co-founder, who has many years of experience in the fields of computer vision, medical image processing and deep learning. We had the same mindset from the beginning and worked well together". Meanwhile Virtonomy can look back on a team of nine people.
"I am one of the most proud of our team," admits Simon, "because the team is the most important thing to me, especially in such a small company like a startup. And a strong team probably needs a startup with goals like Virtonomy's: "We want to become the market leader in the process of data-driven digitization of clinical trials.
Virtonomy's Network: Widely Branched
For Virtonomy, a strong team is the most important, but not the only factor for success. Even more than financial support, the startup is looking for opportunities to establish contacts and networks and to exchange knowledge. Therefore, Virtonomy is part of 5-HT's network, but also of other networks: "We are actually very active in the various digital hubs, such as Nuremberg, Erlangen or the InsurTechHub in Munich. For us, the hubs are a great support, especially in those areas where we are not familiar with or have no contacts yet. We profit a lot from the many years of experience and knowledge of the Digital Hubs. But other startup communities are also a great support for Virtonomy, including Werk1 in Munich.
Virtonomy is therefore not only looking for customers in the field of medical technology, but also for partnerships. "We are always open to discussions and are eager to target new partnerships - from universities to clinics and doctors to hardware manufacturers or completely different sub-areas. Everyone who is active in the field of medical technology is interesting for us".
5-HT Chemistry & Health Newsletter
Want the latest tech and industry news, events, relevant info from the ecosystem and more?
Subscribe to 5-HT Newsletter now Subscribe to 5-HT Newsletter now
Become part of the 5-HT Chemistry & Health
Exchange ideas with innovative startups and future-oriented companies in our ecosystem. We look forward to meeting you!